The Concept of Biological confinement (Bio-confinement) in Closed Systems for Advanced Therapy Medicinal Products production
A novel strategy to easily move ATMPs from and to the isolator
 Recent technological progresses are giving a strong push to the progress of Advanced Therapy Medicinal Products (ATMPs) making them accessible at lower costs for patients in different pathological conditions.
Recent technological progresses are giving a strong push to the progress of Advanced Therapy Medicinal Products (ATMPs) making them accessible at lower costs for patients in different pathological conditions.
The use of closed systems such as isolators for the production of biological drugs in GMP conditions requires the development of strategies that simplify their use.
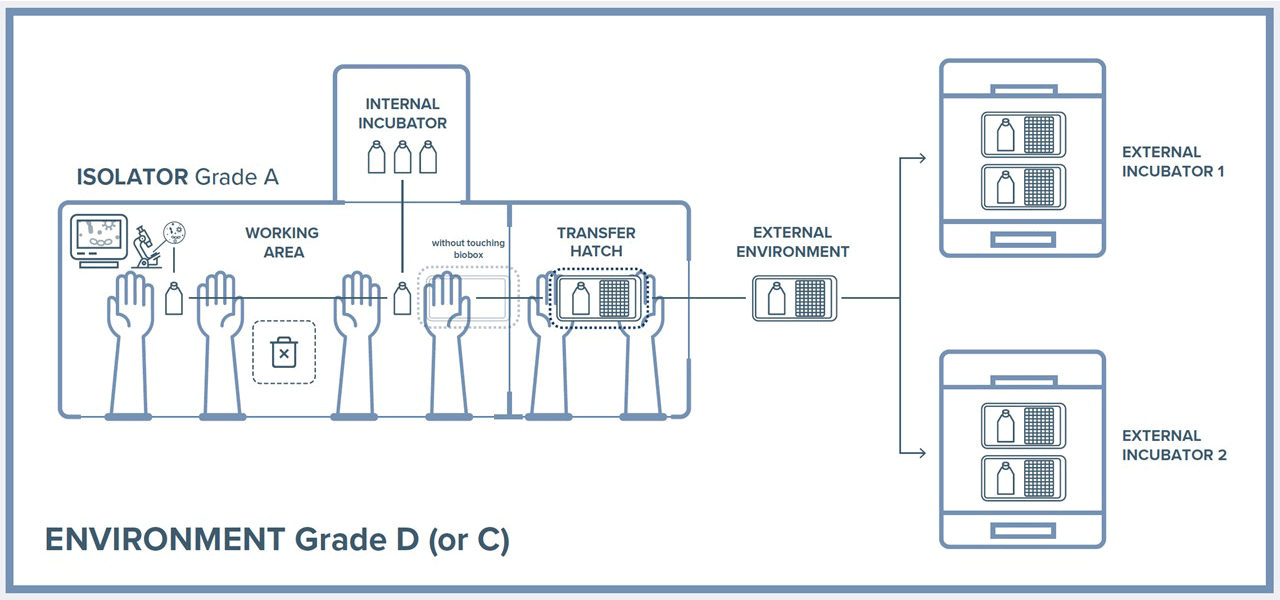
The purpose of this study is the development of a new tool that allows the transfer of cultured cells in a sterile and bioconfined environment (IsocellBIOBOX).
The use of IsocellBIOBOX allows to remove cultured cells from the Isolator and incubate inside standard external CO2 incubators maintaining them in a grade A contained environment.
This solution can keeping cells from different patients safe from cross contamination reducing drastically the equipment costs, compared to other solutions that uses specially designed CO2 Incubators. Cell viability studies, the evaluation of cellular stress as well as the measurement of some physical parameters such as CO2 concentration, demonstrate the total functionality of the IsocellBIOBOX.
In conclusion, IsocellBIOBOX represents an unique, reliable, GMP compliant, low-cost, easy-to-use transfer system that guarantees the bio-confinement of the cultured cells in the same aseptic conditions as in the Grade A work area of the Isolator, in order to be placed into external incubators or other equipment.